新型コロナウイルス感染症(COVID-19)はサイトカインストーム症候群である
| 平野 俊夫 | 国立研究開発法人 量子科学技術研究開発機構 理事長前 大阪大学 総長 |
| COI: | 未確認 |
- 新型コロナウイルス感染症(COVID-19)は、SARS-CoV-2ウイルスにより引き起こされる感染症である。
- 約80%の感染者は無症状か軽症で経過するが、約20%は重症肺炎となり、そのうち30%は致死的な急性呼吸促迫症候群(ARDS)となる。
- 現時点で有効な抗ウイルス薬や、ARDSに対する確立した治療方法はなく、1日も早い治療薬の開発が望まれる。
- SARS-CoV-2はACE2を受容体として感染し、自然免疫系とAngII-AT1Rを介して、NF-kBとSATA3転写因子の活性化を誘導する。STAT3はNF-kBの活性化を増強することにより、IL-6などの炎症性サイトカイン産生を増強する。
- この増幅回路はIL-6アンプと呼称される。関節リウマチなどの慢性炎症性疾患発症に重要な役割を果たし、抗IL-6受容体抗体トシリズマブはこれらの疾患の治療に有効である。
- COVID-19に見られるARDSは、サイトカインストームにより生ずると考えられる。CAR-T治療における致死的な副作用であるサイトカインストームに対して、抗IL-6受容体抗体トシリズマブは有効である。
- 本論文では、サイトカインストームがIL-6アンプの活性化により生じていることを考察するとともに、抗IL-6受容体抗体トシリズマブや、AngII-AT1R阻害薬のCOVID-19治療への可能性について言及する。
はじめに
昨年12月に中国で発症した新型コロナウイルス感染症(COVID-19)は、パンデミック感染症となり、5月23日現在世界中の感染者は521万人、死者は33.8万人に達した。現時点での致死率は6.5%である。
一般の風邪を引き起こすコロナウイルスには、2種類のアルファコロナウイルスと2種類のベーターコロナウイルスが存在する。2003年に流行したSARSウイルス (SARS-CoV-1)や、2015年のMERSウイルス(MERS-CoV)は、これまでの研究でベーターコロナウイルスの仲間であることが明らかになっている[1]。
これらのウイルスの遺伝子情報に基づき、2019年の年末に発生した原因不明の重症肺炎を引き起こすウイルスが、SARS-CoV-1やMERS-CoVと似たRNAウイルスであるベーターコロナウイルスの仲間であることが、短期間に明らかになった[2] [3]。WHOはこのウイルスをSARS-CoV-2と命名した。またSARS-CoV-2で引き起こされるウイルス感染症を、「2019年新型コロナウイルス感染症 (COVID-19)」と命名した。
SARS-CoV-2の遺伝子はSARS-CoV-1と約80%、MERS-CoVとは約50%似ている。また、コウモリのコロナウイルスと約90%似ており、コウモリ由来と考えられている。さらに、SARS-CoV-1の受容体はアンジオテンシン変換酵素2 (ACE2:angiotension converting enzyme 2)であるが[4] [5]、SARS-CoV-2も同じくACE2を受容体とすることが明らかになった[3] [6]。
感染しても約80%は無症状か軽症で経過するが、高齢者を中心に約15%は重症肺炎となり、約5%は致死的な急性呼吸促迫症候群(ARDS: Acute Respiratory Distress Syndrome)になる。また血管炎や血栓症、脳梗塞、心筋障害などを合併するとともに、急性腎機能不全などの多臓器不全を合併することが多い[7] [8]。また、心臓血管疾患、高血圧、糖尿病、慢性肺疾患、慢性腎疾患などの基礎疾患や、加齢、肥満や喫煙などが重症化リスク要因として報告されている。
現時点では、確実に効果のある治療薬は存在せず、一刻も早くワクチンや治療薬を開発するための取り組みが世界中で行われている。特にARDSは致死率が高く、治療方法の開発は緊急の課題である。
SARS-CoV-1やMERS-CoVで引き起こされるARDSではサイトカインストームが生じているが[1]、COVID-19でもInterleukin 1(IL-1), Tumor Necrosis Factor alpha (TNFα) やIL-6などの炎症性サイトカインの産生が増加している[9] [7]。また、重症のARDSにおいては血中IL-6濃度が上昇している [10] [11]。COVID-19におけるARDSはサイトカインストームによって生じていると考えられており、ARDSの治療には単に抗ウイルス薬のみでは不十分で、サイトカインストームを抑制することが必要であると考えられる[12] [13]。白血病の治療に使用されるCAR-T治療における致死的な副作用であるサイトカインストームでは、IL-1 やIL-6が中心的な役割を担っており[14] [15]、IL-6の阻害薬である抗IL-6受容体抗体トシリズマブ(商品名、アクテムラ)が有効であることが示されている[16]。COVID-19における重症肺炎においても抗IL-6受容体抗体トシリズマブの有効性が示唆されている[17] [18] [19]。
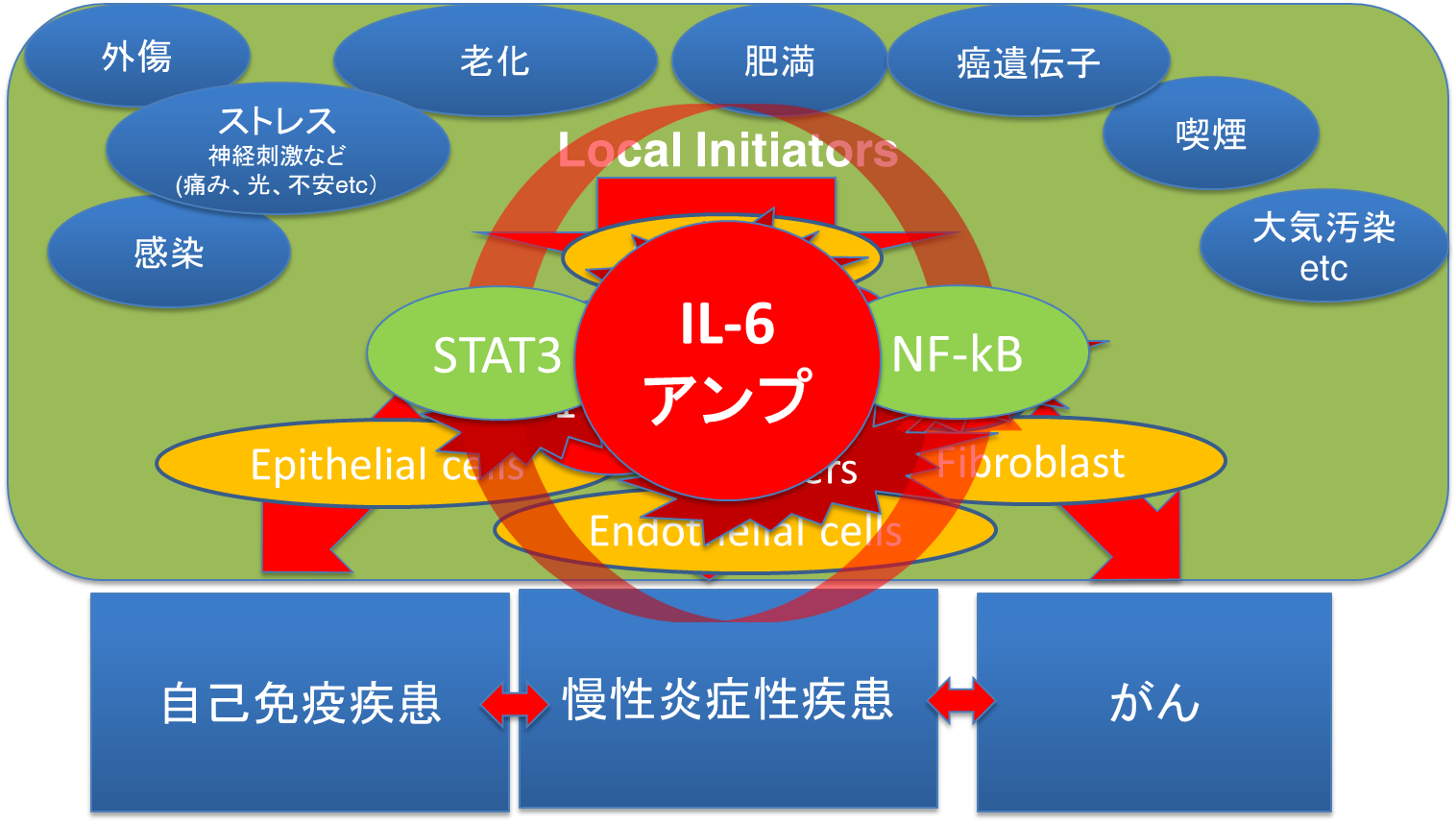
1986年のIL-6発見により[20]、IL-6は免疫反応のみならず、血液系、神経系、内分泌系や初期発生など生体の恒常性維持や慢性炎症性疾患やがんに重要な役割を果たしているサイトカインであることが明らかになった[21]。またIL-6受容体を介してJAKSTAT3活性化経路とSHP2/GAB/ERK/MAPK活性化経路の主たるシグナル伝達系が活性化され、細胞の増殖、生存、分化に関与していることが明らかにされるとともに、IL-6受容体を介するシグナル伝達異常が関節リウマチなどの慢性炎症性疾患を引き起こす[22]。さらに、慢性炎症誘導の基盤として炎症性サイトカイン産生増幅回路であるIL-6アンプ(IL-6 AMP:IL-6 amplifier)の存在が明らかにされた[23]。IL-6 アンプは、気管支・肺胞上皮細胞、線維芽細胞や血管内皮細胞などの非免疫細胞に存在し、非免疫細胞と免疫細胞の相互作用を仲介するとともに、NF-kB経路とSTAT3経路の同時活性化によってNF-kB活性化の亢進を誘導し、種々の炎症性サイトカインやケモカイン、増殖因子などを病態局所にて持続的に産生する[22]。IL-6アンプは、関節リウマチなどの慢性炎症性疾患や自己免疫疾患やがんなどに関与している[24] [25]【図表1】。実際に、抗IL-6受容体抗体トシリズマブが関節リウマチなどの慢性炎症性疾患の治療に有効である[26] [27]。
これらの知見に基づき、COVID-19に発症する致死的な急性呼吸器不全ARDSの発症の仕組みを考察し、その治療としてIL-6-STAT3経路遮断の有効性を考察する。
| 図表1 |
| IL-6アンプは関節リウマチなどの慢性炎症性疾患や自己免疫疾患やがんなどに関与する |
 |
新型コロナウイルス感染は受容体ACE2とTMPRSS2依存的
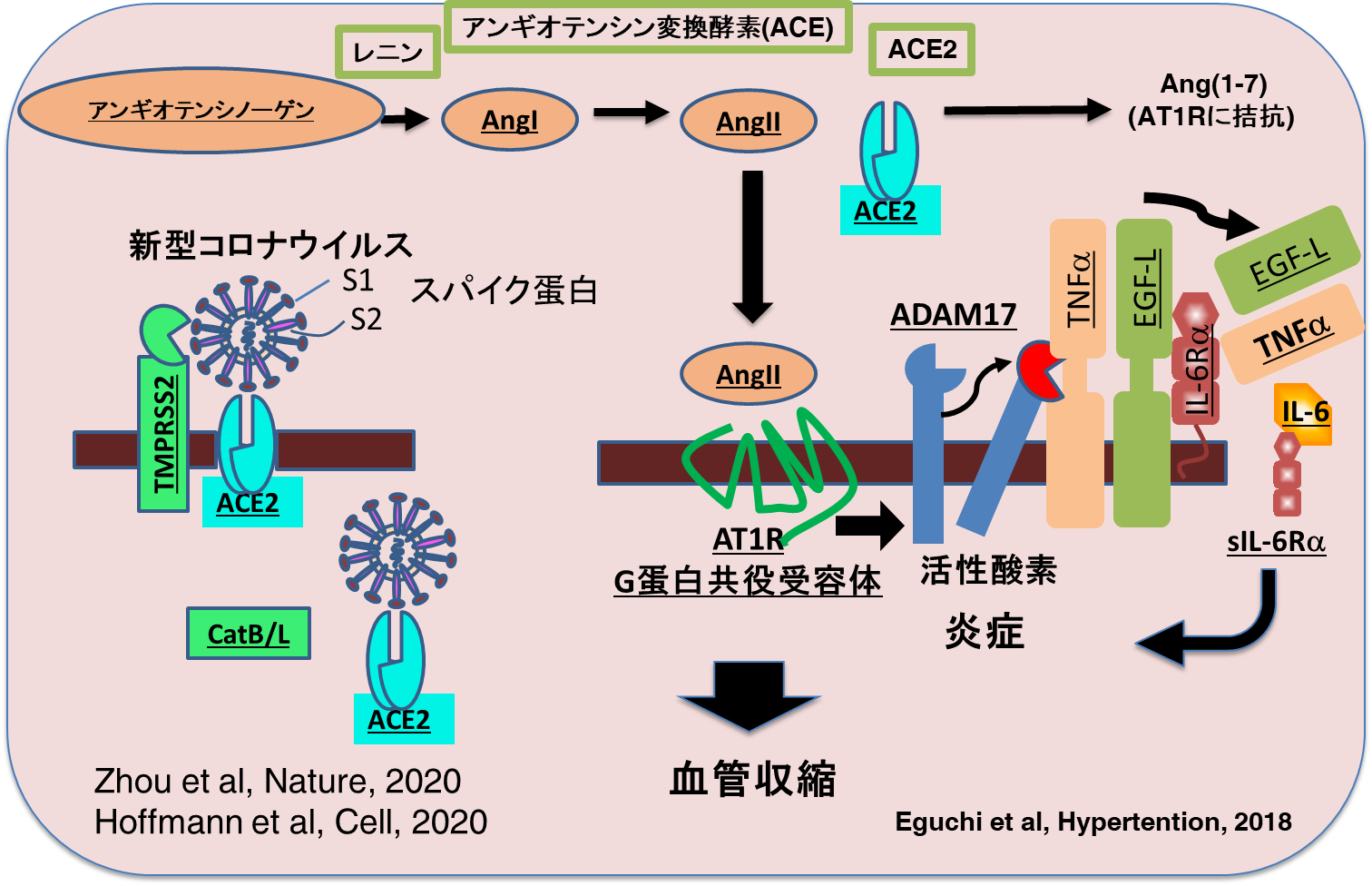
SARS-CoV-2が受容体であるACE2に結合するためにはウイルス膜にあるスパイク蛋白が必要である。SARS-CoV-2のスパイク蛋白の遺伝子配列に基づく構造解析では、SARS-CoV-1のそれと類似していることから、ACE2を受容体としていることが示唆された[2]。細胞を使用したウイルス感染実験やスパイク蛋白の細胞への取り込み実験によりACE2がSARS-CoV-2の受容体であることが明らかになった[3] [6]。ACE2 はI型膜タンパクであり、2型肺胞上皮細胞、心筋細胞、近位尿細管細胞、腸や食道上皮細胞、血管平滑筋細胞、鼻粘膜や口腔粘膜などの扁平上皮細胞などにも発現している。
SARS-CoV-2はACE2を受容体として感染するが、細胞内に入るためにはSARS-CoV-1と同様に、ウイルスのスパイク蛋白が細胞表面に存在するセリンプロテアーゼであるTMPRSS2により切断される必要がある。その後、切断されたスパイク蛋白のサブユニットがウイルス膜と細胞膜の融合を引き起こす結果、ウイルスはACE2とともに細胞内に取り込まれる。実際に、TMPRSS2の阻害剤は、ウイルス感染モデル実験系においてウイルスの細胞内取り込みを阻止した[6]。またACE2に対する抗体もウイルスの細胞内取り込みを阻止した。したがって、ウイルスとACE2との結合を抑制する分子や、TMPRSS2の阻害剤はウイルス感染を抑制する効果が期待され、ウイルス感染の初期には大変有効な治療薬になる可能性がある。このような治療薬の候補としては、ウイルス膜タンパクやスパイクタンパクに対する抗体や、治癒した患者に存在する抗体が期待される。またTMPRSS2の阻害剤としては、すでに日本で膵臓炎の治療薬として承認されているカモスタットやナファモスタットがある[28]【図表2】。
| 図表2 |
| 新型コロナウイルス感染は受容体ACE2とTMPRSS2依存的 |
 |
自然免疫と獲得免疫によりウイルスは排除される
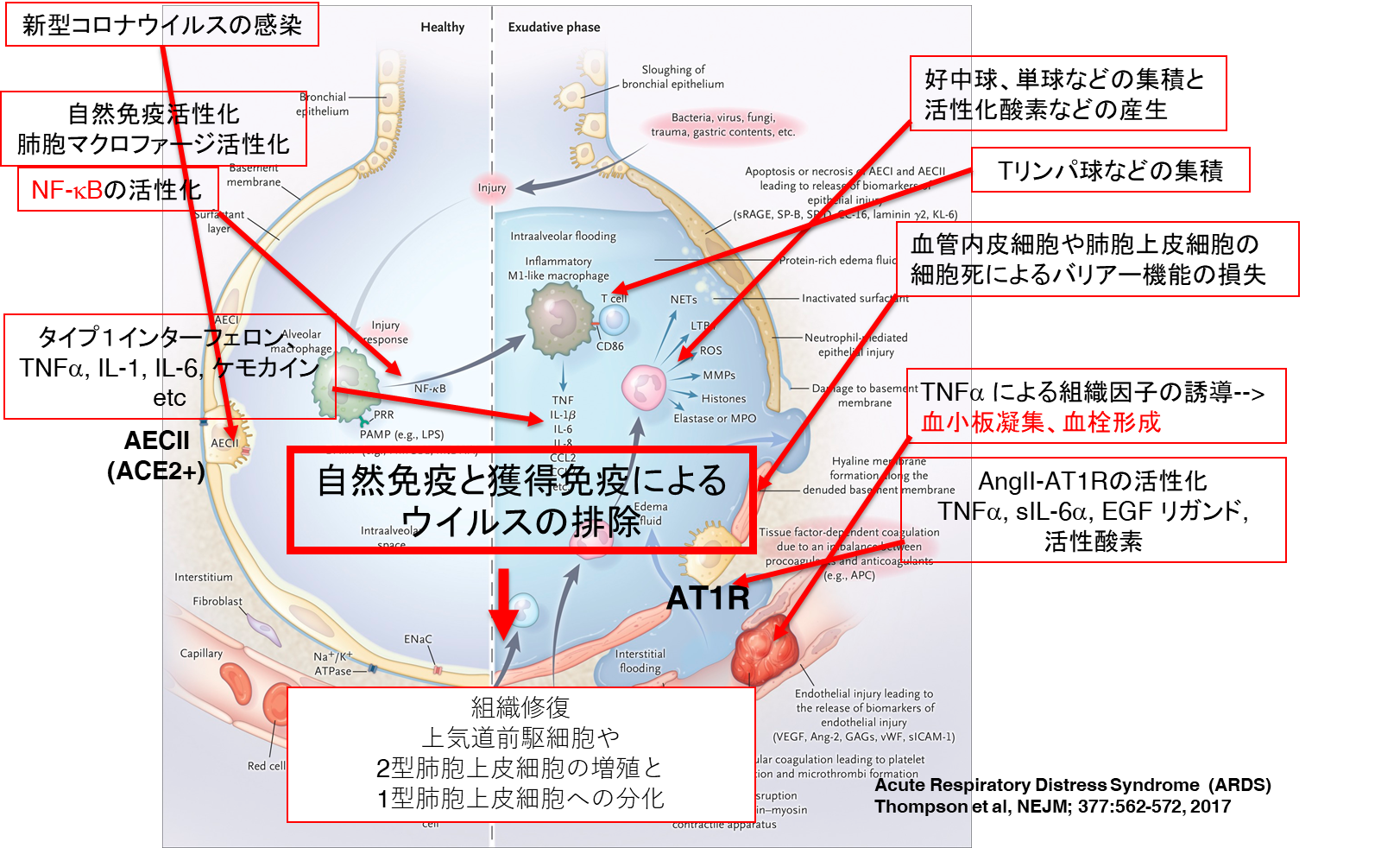
SARS-CoV-1 やMERS-CoVが感染すると、自然免疫の受容体であるPattern Recognition Receptors (PRRs)と呼ばれている分子が活性化され、自然免疫が活性化されることが明らかになっている[1]。SARS-CoV-1では、PRRsである、RIG-1やMDA5が活性化されてMyD88を介して転写因子であるNF-kBが活性化され、TNFα、IL-1、IL-6やケモカインなどが誘導される。また、Interferon regulatory factor 3 (IRF3) とIRF7が活性化され、抗ウイルス活性を有するタイプ1インターフェロンが産生される。TNFαやIL-6はマクロファージや好中球などの自然免疫細胞を活性化し、ウイルスを排除する。
実際IL-6欠損マウスではウイルス感染や細菌感染への抵抗性が低下する[29] [30] [31]。マウスのSARS-CoV-1感染実験において、Myd88欠損マウスではサイトカインやケモカインの産生低下とマクロファージの肺への集積低下が見られるとともに、重篤な肺炎症状を示した[32]。さらに、ケモカインによりTリンパ球、Bリンパ球や樹状細胞が感染局所に引き寄せられて、獲得免疫が活性化される。自然免疫と獲得免疫の免疫反応によりウイルスが排除される。IL-6 はウイルスや細菌感染に対するこれらの免疫反応に重要な役割を果たしている[25]【図表3】。
| 図表3 |
| 自然免疫と獲得免疫によりウイルスは排除される |
 |
実際、ICU治療を必要としなかったCOVID-19患者においては、必要とした患者に比較してスパイク蛋白に対するIgG抗体価は有意に高値を示した。また前者では、スパイク蛋白IgG抗体価の増加はCRP値の低下と相関した[33]。さらに、快復したCOVID-19患者血液中にはSARS-CoV-2特異的CD8+T細胞とCD4+T細胞が認められ、特にスパイク蛋白に対するCD4+T細胞の反応の強さはスパイク蛋白に対するIgGやIgA抗体力価と相関を示した。さらにCOVID-19に感染していない、個人の約40〜60%でSARS-CoV-2反応性CD4 + T細胞が検出された[34]。すなわち、風邪の原因ウイルスであるコロナウイルスに反応するT細胞がSARS-CoV-2とも反応する免疫交差反応の存在が示唆されており、多くの感染者が無症状か軽症で済む要因の1つの可能性がある。また、以下の自然免疫における訓練免疫とも合わせて、想定されている集団免疫閾値が低くなる可能性がある。
日本を含むBCG接種国や地域では、単位人口あたりのCOVID-19発症数や死亡数が少なく、BCG接種が原因の1つである可能性が指摘されている [35] [36] [37]。BCGは自然免疫の強力な刺激効果を有しており、したがってBCG接種は結核以外の感染症にも有効であることが示されている。例えばBCGにより幼児の死亡率が下がるなどである。自然免疫にもTrained Immunity(訓練免疫)と呼称され、獲得免疫における免疫記憶と似た能力がある(あくまでも抗原非特異的である)。BCGにより自然免疫が訓練され、結核菌以外の感染症に対する自然免疫反応も増強されると考えられている [36]。このような現象がCOVID-19にも当てはまれば、上述した獲得免疫における風邪コロナウイルスとの免疫学的交差反応と合わせて、COVID-19による重症化抑制や集団免疫閾値低減化に関与している可能性がある。
このように、SARS-CoV-2感染により自然免疫と獲得免疫が活性化されてウイルスが排除されるとともに、損傷を受けた肺組織などが修復されて治癒にいたる。しかしSARS-CoV-1やMERS-CoV は、PRRsを介するシグナル伝達やタイプ1インターフェロン作用を阻害する様々な分子を作り出して自然免疫を抑制する[1]。また、IL-6-STAT3シグナルはMHCII発現を抑制するが[38]、COVID-19に伴うARDSにおいても、IL-6 増加と単球におけるHLA-DR発現減少、さらにTリンパ球やBリンパ球減少が認められる。抗IL-6受容体抗体トシリズマブでこれらの減少は部分的に快復する[11]。リンパ球減少は獲得免疫の機能低下を招き、ますますウイルスが増殖することになる。そして、免疫反応がウイルスを排除することができずに、肺組織の損傷が拡大していくと、サイトカインストームがおこり重篤なARDSに至る。では、サイトカインストームはどのような機序で生じるかを考察したい。
サイトカインストーム:AngII-AT1R, PRRsとIL-6アンプの共演
上述したように、SARS-CoV-2が細胞に感染すると細胞膜上のACE2発現が減少する。SARS-CoV-1もACE2を受容体として感染するが、それに伴いACE2発現が低下することがすでに明らかにされている[5]。
ACE2 はアンジオテンシII(AngII)をAng(1-7)に変換するので、ACE2の減少によりAngIIが増加する。一方、Ang(1-7)はMasR (Mitochondrial assembly receptor)を介してAT1Rシグナルに拮抗する[39] [40] [41]。このように、細胞膜にあるACE2が減少すると、アンジオテンシン受容体タイプ1(AT1R)を介するAngIIの作用が増強される【図表2】。
AngIIはAT1Rを介して血管収縮のみならず、血管透過性亢進や細胞増殖や炎症誘導作用があり、心臓血管障害やがんに関与する[39] [40] [41]。AT1RはG蛋白質共役受容体で、血管平滑筋、繊維芽細胞、心筋細胞、肺、腎臓、脳など多くの細胞や臓器で発現している。AT1Rはイノシトールトリスリン酸(IP3)やジアシルグリセロールを介してカルシウム濃度上昇やプロテインカイネース Cの活性化を誘導し血管収縮やアルドステロン分泌を誘導するのみならず、活性酸素の産生誘導やADAM17(a disintegrin and metalloprotease 17)という細胞膜上に存在するプロテアーゼを活性化する。その結果、細胞膜に存在するEGF受容体リガンドやTNFαの前駆体が切断されて成熟したEGFリガンドやTNFαが放出される[40]。同様にIL-6受容体sIL-6α(IL-6Rα)もADAM17により切断されて、IL-6Rαも細胞膜から遊離して可溶性IL-6Rα(sIL-6α)が放出される[25]。TNFαはその受容体を介してNF-kBを活性化し、IL-6をはじめとする様々な炎症性サイトカイン産生を誘導するとともに、血管内皮細胞マクロファージに組織因子の発現を誘導し、血栓形成誘導や脳梗塞の原因となる[42]。一方、IL-6はその受容体を介してマクロファージやリンパ球などの免疫細胞に転写因子STAT3を活性化する。血中に放出されたsIL-6RαはIL-6と複合体を形成して、IL-6受容体のシグナル伝達分子であるgp130を発現している様々な細胞(血管上皮細胞、線維芽細胞、肺胞上皮細胞など)に作用して STAT3を活性化する。活性化されたSTAT3はNF-kBに作用して、その活性化をさらに強め、IL-6アンプが活性化される[13]【図表2】【図表4】。
一方、免疫反応がウイルスを排除できない間に、ウイルス感染により肺胞上皮細胞などの細胞死が生じる。大量の死細胞から放出されたダメージ関連分子パターン(DAMP:Damage associated molecular pattern)がPRRsに認識されNF-kBが活性化される。その結果IL-1b、TNFαやIL-6などのサイトカインやケモカイン産生が誘導される。さらに、SARS-CoV-1のN蛋白(Nucleocapsid protein)は、IL-6遺伝子プロモーターに直接またはNF-kBを介して作用することによりIL-6遺伝子発現を誘導する[43]【図表4】。
| 図表4 |
| サイトカインストーム:AngII-AT1R, PRRsとIL-6アンプの共演 |
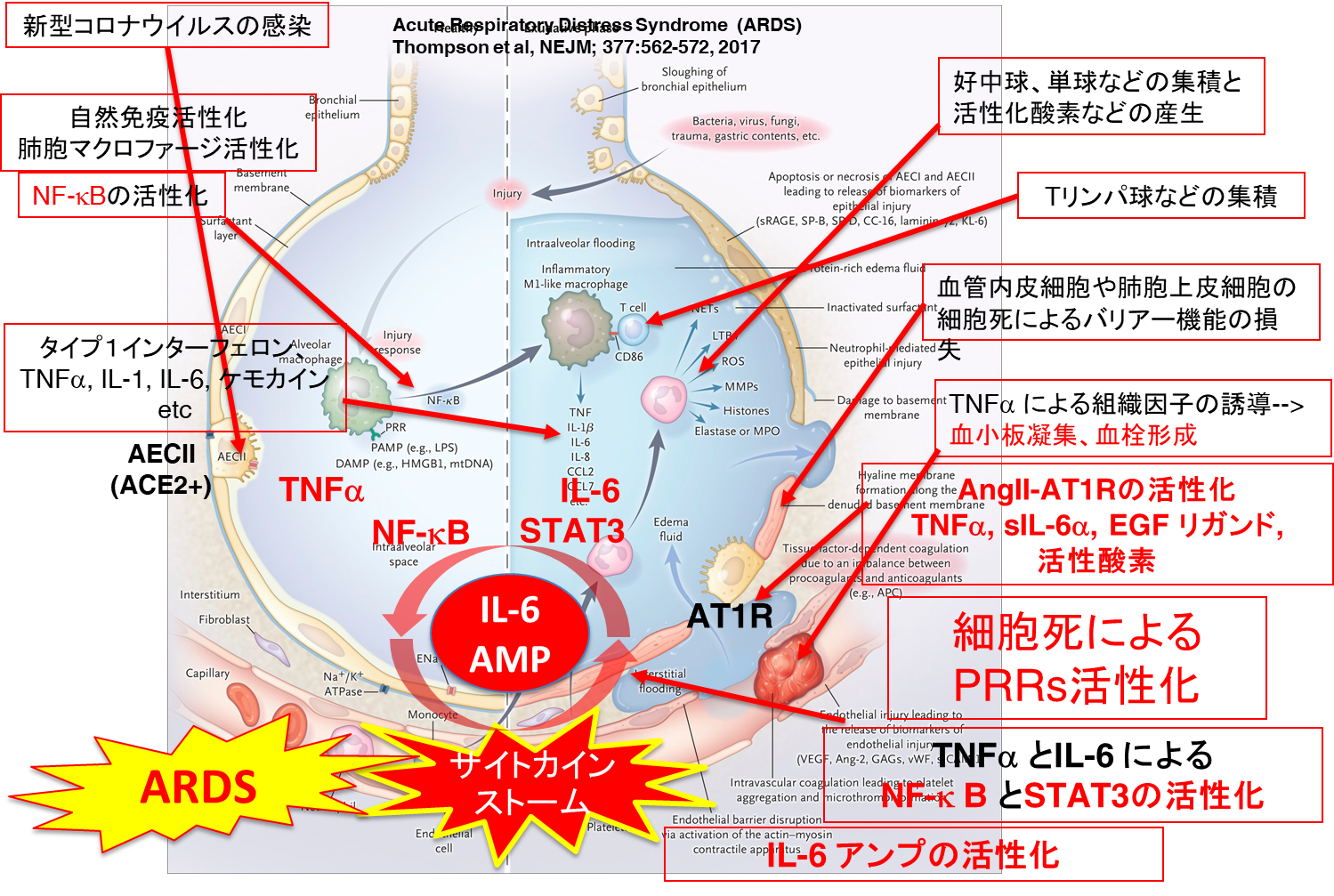 |
このように、自然免疫を介するシグナル伝達系とAngII-AT1Rを介するシグナル伝達系が協調して、STAT3によるNF-kB活性化が持続的に亢進する。すなわちIL-6アンプが活性化されて、大量の炎症性サイトカインやケモカインなどが産生されて、サイトカインストームに至ると考えられる[13]。
実際に、SARS-CoV-1によるARDSが、ACE2リコンビナントタンパク投与やAT1R阻害剤で阻止できる[5]。さらに、トリインフルエンザウイルスA(H7N9)感染で生じるARDSにおいてもACE2-AngII-AT1Rが重要な役割を果たしており、AT1R阻害剤がARDSを抑制した[44]。SARS-CoV-2によるARDSにおいても抗IL-6受容体抗体トシリズマブの有効性が示唆されている[17] [18] [19]。
重症化リスク要因としては、心臓血管疾患、高血圧、糖尿病、慢性肺疾患、慢性腎疾患などの基礎疾患や、加齢、肥満や喫煙などが報告されている。加齢に伴い免疫機能は低下するので、一般的に感染症に対するリスクは上昇する。加齢を含め、これらのリスク要因は少なからず慢性炎症と関連があり、IL-6アンプ活性化ベースラインを引き上げている可能性がある。実際、加齢に伴いIL-6は上昇しており、IL-6アンプの活性化は起こりやすくなっていることが考えられる。加齢、肥満や喫煙は慢性炎症を誘導し、糖尿病、心臓血管疾患などのIL-6が関与している慢性炎症疾患や悪性腫瘍と密接な関連がある。また、これらのリスク要因はACE2-AngII-AT1Rとの関連もある。サイトカインストームとどのような関連があるかはさらなる解明が必要。
サイトカインストームはウイルス感染に限定されない
サイトカインストームを伴うARDSは、ACE2受容体とするSARS-CoV-1やSARS-CoV-2のみならず、MERS-CoVやインフルエンザウイルスなどのウイルス感染や敗血症などでも生じる。さらには誤嚥性肺炎のように胃酸や人工呼吸器などによる肺の損傷でも生じる[45]。また白血病の治療で行われるCAR-T治療においても生じる。これらに共通しているのは、大量の細胞死である。死細胞から放出されるDAMPsがPRRsを活性化しIL-6アンプが活性化されると考えられる。実際にCAR-T治療における致死的なサイトカインストームはIL-1やIL-6が関与しており[14] [15]、その治療には抗IL-6受容体抗体トシリズマブが有効である[16]。また、肺炎のみならず、敗血症や胃酸、あるいは肺の外傷によって引き起こされるARDSモデルマウス実験においても、ACE2-AngII-AT1Rシグナル伝達系が重要な役割を果たしている[46]。興味深いことに、ARDS発症とその重症化にACE遺伝子多型が関与していることが報告されている[47]。さらに、胃酸によるARDSやSARS-CoVやインフルエンザウイルスH5N1で誘導されるARDSマウスモデル実験においてもPRRsの一種であるTLR4を介するマクロファージの活性化が関与している[48]。さらに、敗血症ラットモデル実験で誘導されるARDSや腎機能不全が、抗IL-6受容体抗体がNF-kB 活性化を抑制することにより抑制することが示されている[49]。
まとめ
このように、ウイルスや細菌感染のみならず外傷により引き起こされる肺の損傷はACE2-AngII-AT1Rシグナルを活性化するとともに、自然免疫系を活性化し、その結果としてTNFα/IL-1β-NF-kBとIL-6-STAT3が相乗的に働きIL-6 アンプ活性化を介して制御されないサイトカイン産生を誘導してサイトカインストームに至ると考えられる。したがって、COVID-19に見られるARDSの治療にはIL-6-STAT3阻害剤やAngII-AT1R阻害剤が有効であると考えられる。ただし、IL-6などのサイトカインは抗ウイルス活性があるので、感染初期に投与すると逆効果になると考えられるので、投与時期は血中IL-6濃度やD-ダイマーなどの組織損傷マーカーなどを指標に慎重に選ぶ必要がある[11] [50]。これらの阻害剤はウイルスや細菌のみならず外傷などで引き起こされるサイトカインストームが原因のARDSはもちろんのこと腎機能不全など他の臓器不全にも効果が期待できる。
[引用文献]
- de Wit, E., et al., SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol, 2016. 14(8): p. 523-34.
- Lu, R., et al., Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 2020. 395(10224): p. 565-574.
- Zhou, P., et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 2020. 579(7798): p. 270-273.
- Li, W., et al., Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 2003. 426(6965): p. 450-4.
- Kuba, K., et al., A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med, 2005. 11(8): p. 875-9.
- Hoffmann, M., et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 2020. 181(2):271-280
- Huang, C., et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 2020. 395(10223): p. 497-506.
- Cao, X., COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol, 2020. 20(5): p. 269-270.
- Chen, N., et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 2020. 395(10223): p. 507-513.
- Chen, X., et al., Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis, 2020.
- Giamarellos-Bourboulis, E.J., et al., Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe, 2020. doi: 10.1016/j.chom.2020.04.009
- McGonagle, D., et al., The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev, 2020: p. 102537.
- Hirano, T. and M. Murakami, COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity, 2020. 52(5): p. 731-733.
- Norelli, M., et al., Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med, 2018. 24(6): p. 739-748.
- Giavridis, T., et al., CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med, 2018. 24(6): p. 731-738.
- Neelapu, S.S., et al., Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nat Rev Clin Oncol, 2018. 15(1): p. 47-62.
- Xu, X., et al., Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A, 2020. 117(20):1097-10975
- Luo, P., et al., Tocilizumab treatment in COVID-19: A single center experience. J Med Virol, 2020. doi: 10.1002/jmv.25801
- Alattar, R., et al., Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol, 2020. doi: 10.1002/jmv.25964
- Hirano, T., et al., Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature, 1986. 324(6092): p. 73-6.
- Hirano, T., Interleukin 6 and its receptor: ten years later. Int Rev Immunol, 1998. 16(3-4): p. 249-84.
- Hirano, T., Interleukin 6 in autoimmune and inflammatory diseases: a personal memoir. Proc Jpn Acad Ser B Phys Biol Sci, 2010. 86(7): p. 717-30.
- Ogura, H., et al., Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity, 2008. 29(4): p. 628-36.
- Murakami, M., et al., Disease-association analysis of an inflammation-related feedback loop. Cell Rep, 2013. 3(3): p. 946-59.
- Murakami, M., D. Kamimura, and T. Hirano, Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity, 2019. 50(4): p. 812-831.
- Nishimoto, N., et al., Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood, 2005. 106(8): p. 2627-32.
- Maini, R., et al., Double‐blind randomized controlled clinical trial of the interleukin‐6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis & Rheumatism, 2006. 54(9): p. 2817-2829.
- Yamamoto, M., et al., Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay. Antimicrob Agents Chemother, 2016. 60(11): p. 6532-6539.
- Kopf, M., et al., Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature, 1994. 368(6469): p. 339-42.
- Ladel, C.H., et al., Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun, 1997. 65(11): p. 4843-9.
- Dienz, O., et al., Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol, 2012. 5(3): p. 258-66.
- Sheahan, T., et al., MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog, 2008. 4(12): p. e1000240.
- Sun, B., et al., Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect, 2020. 9(1): p. 940-948.
- Grifoni, A., Targets of T cell response to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexpected individuals. Cell, 2020: DOI: https://doi.org/10.1016/j.cell.2020.05.015
- Sato, J. If I were North Amerian/West European/Australian, I would take BCG vaccination now against the novel coronavirus pandemic. https://www.jsatonotes.com/2020/03/if-i-were-north-americaneuropeanaustral.html
- Netea, G. N, et al, Trained Immunity: a Tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell, 2020. https://doi.org/10.1016/j.cell.2020.04.042.
- Miyasaka, M., Is BCG vaccination causally related to reduced COVID-19 mortality? EMBO Mol Med, 2020. doi: 10.15252/emmm.202012661
- Kitamura, H., et al., IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity, 2005. 23(5): p. 491-502.
- Xu, J., et al., The ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Pleiotropic Roles in Cancer. Front Physiol, 2017. 8: p. 276.
- Eguchi, S., et al., Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension, 2018. 71(5): p. 804-810.
- Vaduganathan, M., et al., Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med, 2020. 382(17): p. 1653-1659.
- Grover, S.P. and N. Mackman, Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol, 2018. 38(4): p. 709-725.
- Zhang, X., et al., Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology, 2007. 365(2): p. 324-35.
- Yang, P., et al., Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep, 2014. 4: p. 7027.
- Thompson, B.T., R.C. Chambers, and K.D. Liu, Acute Respiratory Distress Syndrome. N Engl J Med, 2017. 377(19): p. 1904-1905.
- Imai, Y., et al., Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature, 2005. 436(7047): p. 112-6.
- Marshall, R.P., et al., Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med, 2002. 166(5): p. 646-50.
- Imai, Y., et al., Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell, 2008. 133(2): p. 235-49.
- Ibrahim, Y.F., et al., Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down-regulation of NF-kappaB/JNK: a possible role of P-glycoprotein. Inflammopharmacology, 2020. 28(1): p. 215-230.
- Wu, C., et al., Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med, 2020. doi: 10.1001/jamainternmed.2020.0994
